Why does UKHSA use Animal Testing?
Fig 1. UKHSA Research
Using Animal Testing in UKHSA Research
The UKHSA recognises that the use of animals in research is a sensitive and complex issue, and we are committed to being transparent about our use of animals in research. We believe that it is important to communicate openly with the public about the role of animal research in advancing scientific knowledge and improving human health (Fig.1).
The biological similarities between humans and other species means that they can, on some occasions, be the only effective model for research where responses to agents is too complex to be modelled in any other way. Such animal models provide key scientific evidence that informs interventions, policies and responses to local, national and international health security.
The UK Health Security Agency (UKHSA) uses animal tesing in specific and carefully considered circumstances to support our public health research and protection responses to infectious diseases, vaccines and evironmenal hazzards. Within our vaccine and therapy development, animals are used to assess the safety and efficacy of new treatments for diseases like influenza, HIV/AIDS, tuberculosis, hepatitis C, meningococcal disease, neonatal sepsis and other infectious diseases.
Animal Research at UKHSA
Where do we use animals in our research?
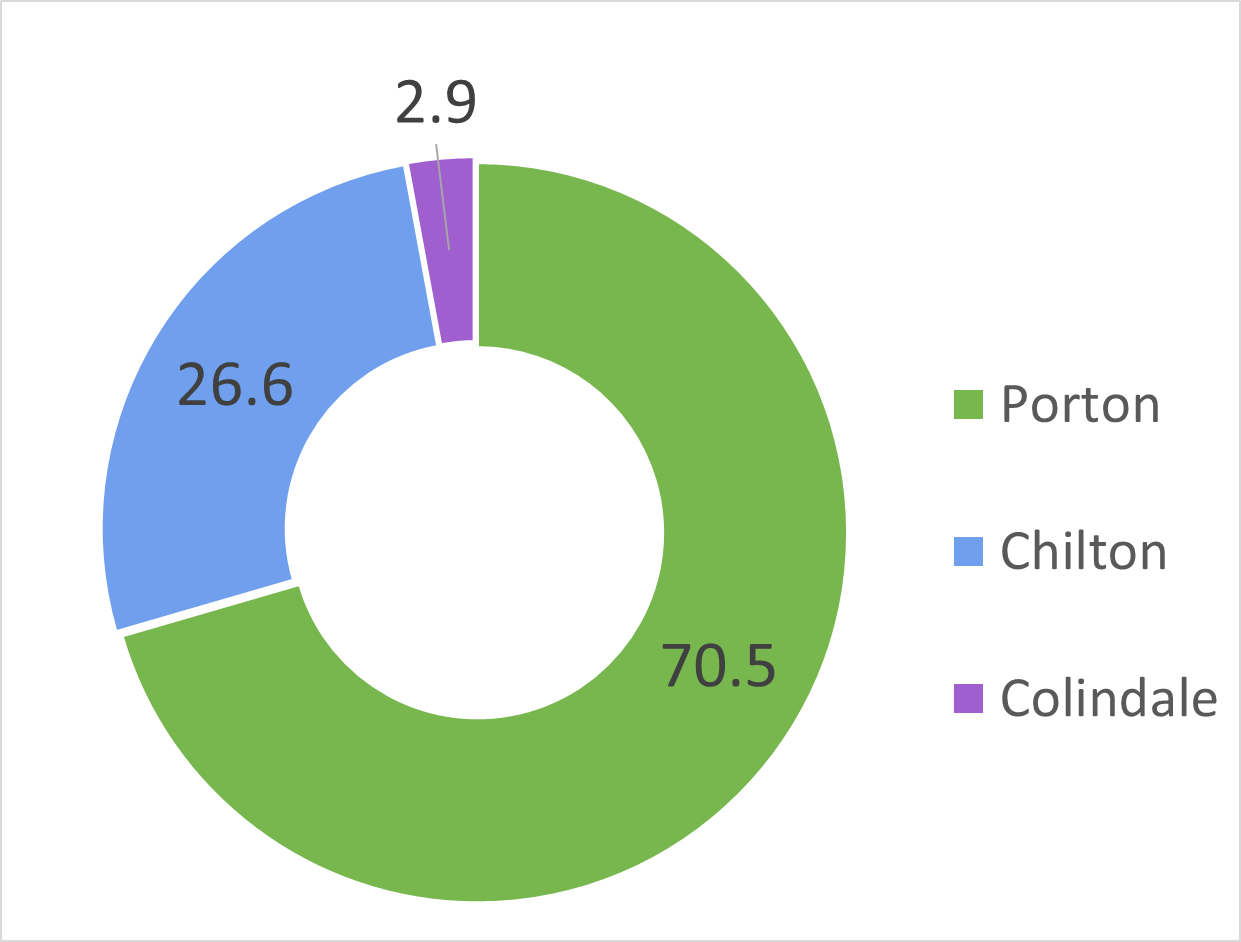
Fig 2. UKHSA Research
Annual UKHSA animal usage over the last 5 years by site. Values represent the annual average percentages of average annual total of 5695 animals from 2017 to 2021.
Where do we use animals in our research?
The animal work conducted at the 3 UKHSA sites Fig.2 provides an essential capability for health security as well as opportunities for scientific advancement aligned to UKHSA’s mission of interest to R&D funders internationally. UKHSA has substantial experience in assisting in the development of countermeasures to protect the population from health threats.
UKHSA Porton also includes Porton Biopharma Limited (PBL), which focusses on the quality-assured development of life-saving biopharmaceuticals. PBL manufacture the licenced childhood leukaemia therapy Erwinase, and the UK’s licenced anthrax vaccine.
Add link hereWhy do we use our animals in our research?
How do we use animals in our research?
More specifically, animals are used in UKHSA reserach to:
- improve methods of diagnosis and to identify the severity of new and emerging pathogens.
- support the development of, and access to, new vaccines or therapies for the main public health threats and emerging or re-emerging diseases, including those on the UKHSA list of pathogens characterise the effectsof low does radiation on colon cancer, leukaemia, cateract or cardiovascular disease development.
- investigate the additional positive effects of sunlight beyond the benefits of vitamin D.
- identify the potential toxicity of inhaled particles, including particulate matter and nanomaterials.
- inform policies, interventions and messaging campaigns to better protect human health.
- improve the management of casualties in acute radiation, chemical and other environmental incidents.
Further details of animal studies conducted at UKHSA can be viewed Here
How many animals do we Use in our research?
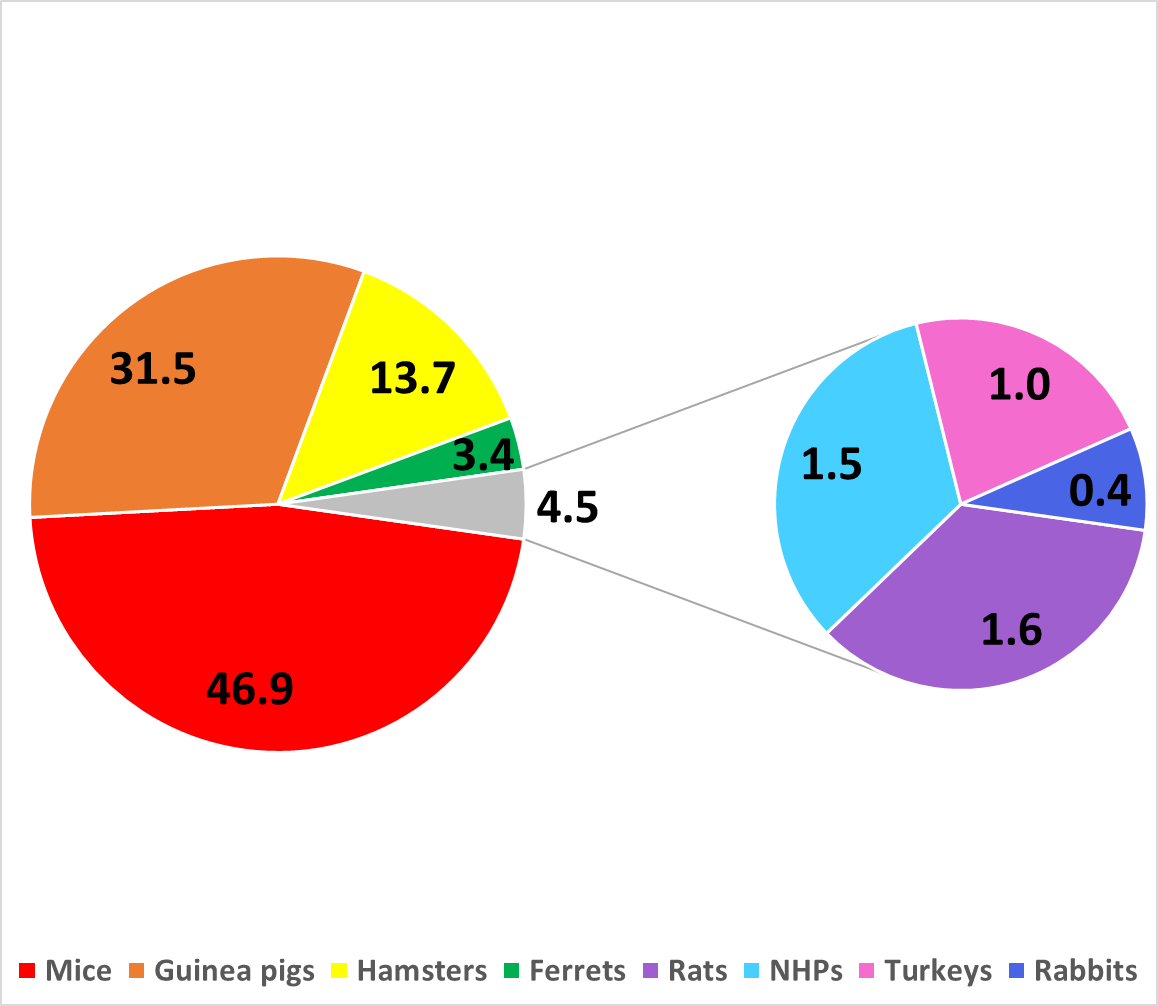
Fig 3. Annual UKHSA animal usage by species
Annual UKHSA animal usage over the last 5 years by site. Values represent the annual average percentages of average annual total of 5695 animals from 2017 to 2021.
How many animals do we use?
Across the 3 sites using animals in their research, an average of 3400 animals are typically used per year. This is less than 6% of the usage of the top 10 UK organisations that performed the most animal research, which reported the use of over 60,580 (10th) to 223,787 (1st) animals in 2023. Click for more information on how UKHSA compares to other UK organisations, and how the UK compares to other European nations.
The majority of the animals used each year are mice (47%), followed by guinea pigs (31.5%), hamsters (14%), ferrets (3%), and non-human primates, rabbits, rats and turkeys (4.5% total) (Figure 2A). The vast proportion of these animals suffer no, mild or moderate harm. UKHSA Porton uses the most animals (70%), followed by UKHSA Chilton (27%) and UKHSA Colindale (3%) (Figure 3). Click for more information on UKHSA animal use for 2023 and previous years.
How is animal research regulated in the UK?

How is animal research regulated in the UK?
Use of animals for research in the UK is governed by the Animals (Scientific Procedures) Act 1986. This stipulates that animal research can only be performed under a specified Project Licence (PPL), within an organisation with a Place Establishment Licence (PEL), and by individuals holding a Personal Licence (PIL). ASPA is implemented and regulated by the Animals in Science Regulation Unit (ASRU) with the Home Office. More information on ASPA and its operation can be found here.
One requirement of the ASPA is to have an Animal Welfare and Ethical Review Body (AWERB) to ensure that the use of animals is carefully considered, justified and performed to high standards; all opportunities for reduction, refinement and replacement (the 3Rs) are implemented; and the four commitments of the Understanding Animal Research (UAR) Concordat on Openness are upheld.
The PEL holder at each site has overall responsibility for the implementation of ASPA and the operation of the AWERB. The 3 AWERBs function locally but work collaboratively to share best practice, reporting quarterly into the UKHSA Science Governance Committee. More information can be found on UKHSA AWERB terms of reference.
How UKHSA strives to implement the 3'Rs
Replacement
Replacement is the use of non-animal alternatives in place of using animals. While suitable non-animal alternatives that completely model the complex human health responses to environmental hazards or pathogens are currently available, UKHSA is working on multiple new approach methodologies (NAMs). This includes Organ on a Chip.‘organ on a chip’ systems to help develop vaccines, drug and therapeutics against a broad range of other dangerous infectious diseases including MERS and Influenza virus. In the long term, we anticipate that this research will accelerate the rate of development of novel medicines. In addition, we are developing and validating new cell-based tests to support animal-free chemical safety regulation.
Reduction
Reduction involves minimising the number of animals used in research while maintaining the validity of the data collected, ensuring that no animals are used unnecessarily. Throughout the lifetime of our research projects using animals, we continually review and employ improved statistical approaches, which have helped us to use less animals than originally planned. Wherever possible we also share animal tissue and results with internal and external collaborators to maximise the data generated from animals and allow new technologies to add value to existing samples, thereby preventing duplication of experiments.
At UKHSA we use in vitro screening of potential countermeasures wherever possible and we have established a comprehensive archive of animal blood and tissues that can be shared across the organisation to develop new technologies or apply new analysis that will enhance our interpretation of previously conducted studies, removing the need for additional animals
Refinement
Refinement is centred around improving the experience of the research animal by minimising pain, suffering, distress and harm. At UKHSA the care and conditions for animals in research are continually refined to minimise harm and distress as much as possible. Practices are regularly assessed and updated to enhance animal well-being at every opportunity. This has involved ensuring group housing for social animals and developing novel and exciting enrichment activities for the wide variety of species we use. For example, ……. (could ask Susan and others to describe the enrichment initiatives and provide pictures?).
Examples of refinement include housing animals in socially compatible groups wherever possible, provision of enrichment appropriate to the species to encourage natural behaviours and adopting sampling techniques in line with advice from bodies such as the National Centre for the Replacement, Refinement and Reduction of Animals in Research NC3R's through regular liaison. In addition, we have refined studies through the use of advanced medical imaging that provides critical data whilst being non-invasive
Click here for more information